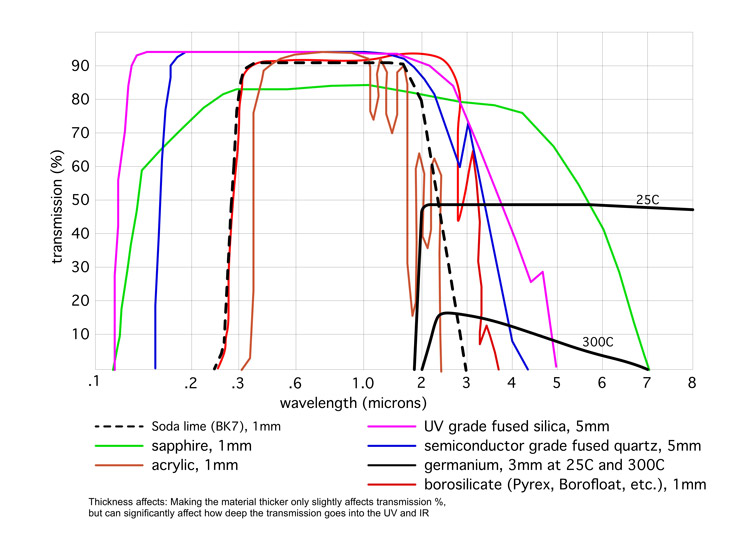
Transparent Materials Comparison
GLASS VS CRYSTALINE MATERIALS?
Glass is an amorphous solid which means its atoms are randomly oriented. Whereas, a crystalline material may contain the exact same type and quantity of atoms as glass, but the atoms are ordered in rigid, well-defined patterns. Fused quartz glass vs. crystalline quartz is a good example of the same type of atoms (SiO2) structured in such a way that one orientation forms a glass and another forms a crystal.
Crystalline materials are solid and keep their shape until they reach a very specific temperature, at which time they become fluid. In contrast, all glasses, by definition, are always a fluid. The viscosity of glass is determined by the temperature of the glass; even at room temperature glass is moving, albeit very slowly. When product tolerances are very tight and temperatures are elevated, a crystalline material is superior to glass because it will maintain its shape at any temperature below the melting point. Conversely, glass will change shape over time, regardless of the melting point and especially at elevated temperatures.
Both crystalline solids and glasses are normally colorless in the pure state. Impurities are actually what give sapphire and glass their color. The impurities also change the mechanical, thermal, and optical properties of both material types, especially for glass.
SAPPHIRE
Sapphire is a single crystal – Aluminum Oxide (Al2O3) – which is colorless and optically clear. Synthetic sapphire is grown in boules (bulk pieces) no larger than 300mm in diameter. It can also be grown into shapes such as sheets, ribbons, domes and tubes with very smooth surface qualities, high purity and optical translucence. When used “as grown” there is very little need for grinding and polishing.
WHY SAPPHIRE?
- Mechanically second only to diamond. One of the hardest and most scratch resistant materials available. The high modulus of elasticity and high tensile strength make it extremely wear, abrasion and impact resistant.
- Colorless optical characteristics are superior to any standard glass, with up to a 98.5% transmission and a transmission window from 190 nanometers in the UV to 5 microns in the IR.
- No solarization in high-radiation systems.
- High dielectric constant and low loss tangent makes it a great electrical insulator and low loss long wavelength window.
- Thermally very stable. Does not lose any of its mechanical and optical qualities from cryogenic to over 2000C.
- Thermal conductivity greater than other optical materials and most dielectrics.
- No surface damage and devitrification due to extreme thermal cycling Does not sag or slump at very elevated temperatures.
- Highly corrosion resistant. More resistant to corrosive chemicals than most standard hard materials available.
WHY NOT SAPPHIRE?
- Cost of material. Sapphire often costs more than other refractory materials. But not always! Quantity and geometry play a major role in the cost of the final product, especially with smaller products where the labor is the primary cost.
- Cannot be bent, molded, drawn or melt-fused like glasses and metals. Sapphire can only be ground and mechanically polished.
- Size limitations. The maximum size of a sapphire product cannot exceed the largest boule that can be grown. Therefore, maximum part size cannot exceed 300mm for two of the dimensions.
- Larger pieces can be thermally shocked and broken if not heated uniformly.
FUSED QUARTZ AND FUSED SILICA GLASS
Fused quartz and fused silica are the amorphous form of quartz. Fused quartz is made from purifying and melting natural crystalline quartz, usually natural quartz sand. Fused silica is a purer version of fused quartz that is made from various silicon gasses. Chemically known as SiO2, silica is “pure” glass. All other commercial glasses are SiO2 with other dopants added to lower the melting temperature and modify optical, thermal and mechanical characteristics.
WHY FUSED QUARTZ AND FUSED SILICA GLASS?
- Extremely low coefficient of expansion, making it far more shock resistant than any other
refractory material - Best transmission characteristics of any standard glass: 220 nanometers to 3 microns for
standard semiconductor-grade fused quartz, and 175 nanometers to 3 microns for many types
of fused silica - Highest temperature characteristics of any glass. A continuous maximum of 900C to 1100C,
depending on the size and shape of the part. Can be used up to 1400C for short periods of time - Very low dielectric constant and the lowest loss tangent of almost all known materials
- Very low thermal conductivity
- Can be melted, bent, fused, drawn and welded into tube and rod forms
- Can be ground and polished
- With the right technology, can be molded, slumped and drawn into fiber, tube and rod shapes
- Harder than most glasses
- Can be made into any shape and relatively large sizes
- Excellent resistance to non-fluorinated acids, solvents and plasmas
- Excellent for containing many high-purity chemicals
- Less expensive than sapphire for larger parts
WHY NOT FUSED QUARTZ AND FUSED SILICA GLASS?
- Much more costly than other standard glasses
- Can sag and slump over time at elevated temperatures
- Surface devitrifies over time when temperature is cycled at high temperatures
- Breaks down with some caustics, fluorinated acids and plasmas
- Can solarize in high radiation environments
- Due to the high melting point, fabrication costs for melting and blowing are much higher than other standard glasses
- Standard shapes are tubes and boules. Does not come in standard sheets like borosilicate
and soda lime glasses. In other words, other than tubes, all fused quartz and silica products
must be ground and polished from a large block.
BOROSILICATE GLASS
Borosilicate glass is an “engineered” glass developed specifically for use in laboratories and applications where thermal, mechanical and chemical conditions are too harsh for standard, household-type glass. Some common names of borosilicate are Pyrex ™ by Corning and Duran ™ or borofloat ™ by Schott Glass. Like most glasses, the main component of borosilicate glass is SiO2 with boron and various other elements added to give it its excellent qualities.
WHY BOROSILICATE GLASS?
- Much easier to hot-work than quartz, making fabrication less costly
- Material cost is considerably less than fused quartz
- Compared to all glasses, except fused quartz, it has a low coefficient of expansion (three times
less than soda lime glass). Useful for cooking, heating and other thermal environments, without
the risk of breakage due to thermal shock. - Like soda lime glass, the float process is used to make relatively low-cost, optical-quality
sheet borosilicate glass in a variety of thicknesses (less than 1mm to over 25mm thick) - Easily moldable (compared to quartz)
- Minimum devitrification when molding and flame working. High-quality surfaces can be
maintained when molding and slumping - Thermally stable up to 450C for continuous use, and up to 600C for short periods
- More resistant to non-fluorinated chemicals than household soda lime glass
- Mechanically stronger and harder than soda lime glass
WHY NOT BOROSILICATE GLASS?
- Material will not maintain its shape if exposed to thermal conditions greater than 450C for long periods of time.
- Cost. Borosilicate is usually 2 to 3 times more expensive than soda lime material.
- Not as thermal shock resistant as quartz, which has a coefficient of expansion ~60x less.
- For high purity chemicals, some minor leaching can occur over time, especially if exposed
to some acidic or basic chemicals - Cannot be fully tempered like soda lime glass
SODA LIME GLASS
Soda lime glass is the “original” glass, appearing in its most basic form thousands of years ago. Commonly called float glass, it is often formed by floating soda lime glass on a bed of molten tin. It is also known as crown glass, a high-silica form of soda lime that was historically used for windows. Soda lime glass is composed of SiO2: sodium oxide (soda) and calcium oxide (lime). About 90% of the glass used in the world – including most windows, dinnerware, art and lighting products – is one of 50,000 variations of soda lime glass
Although soda lime glass typically has a green or blue-green tint to it, the iron content can be reduced to the point where the glass becomes crystal clear, also known as “water white.”
WHY SODA LIME GLASS?
- Inexpensive and easy to mass produce
- Low melting temperature; maintains softness for a long time. This allows for long working
times and faster production rates. - Easily “floated,” making it a very low-cost, flat (float), optically clear sheet glass.
- Softer than borosilicate and quartz, making scribe cutting easier and faster.
- Because of its high coefficient of expansion, it is easily tempered. Tempered glass is up
to 3 times stronger than non-tempered glass and it crumbles when broken — a good
(and often required) safety feature.
WHY NOT SODA LIME GLASS?
- High coefficient of expansion, thus, very poor thermal shock resistance. Only good in
thermal environments where heating is uniform and gradual - Will sag easily at relatively low temperatures
- Does not come in as many stock thicknesses as borosilicate
- Many chemicals will leach the glass over time, making it unsuitable for pure chemical applications
- Not as scratch resistant as borosilicate and quartz
OTHER MATERIALS
GLASS CERAMIC
Glass ceramic is a clear, light amber-tinted or opaque black material. It comes stock in only 3mm, 4mm and 5mm thicknesses. It has many of the thermal performance properties of fused quartz including a very low coefficient of expansion. For fabrication capabilities, it is more like sapphire, as it cannot be melted or welded. It is not nearly as good for optical applications as fused quartz, sapphire, soda lime or borosilicate. However, it is ideal for furnaces and fireplaces with windows where the tint of the glass ceramic is not a cosmetic problem.
EXOTIC GLASS
Exotic Glass is used in specialized applications where more the common glasses do not meet the optical, electrical or mechanical requirements. Many of these exotic glasses can be molded to at least 2 dimensional curves. Special care may be necessary for some glasses because of a tendency to devitrify or crystallize when softened and then re-cooled.
TRANSMISSION CURVES





